Which of the Following Would Have the Highest Vapor Pressure
Among the choices liquid ether molecules more readily escape as vapor so that the boiling point is reached sooner. The vapor pressure of ethyl alcohol C2H5OH at 35 C is 1005 mm Hg.

Which Molecules Have Higher Or Lower Vapor Pressure Youtube
Which one of the liquids would you expect to have the highest vapor pressure at room temperature.

. July 23 2021 July 23 2021 thanh. A 10 M solution of ionic compound sodium chloride NaCl. The graph shows that propanone has the greatest vapor pressure at any given temperature compared to the other three liquids while ethanoic acid has the lowest vapor pressure at any given temperature compared to the other three liquids.
Thus at room temperature the substance with the lowest boiling point will have the highest vapor pressure easiest to get into the gas phase. Due to its smaller size ethanol exhibits weaker dispersion forces than diethyl ether. Chemistry 03072019 0700 brooke0713.
The freezing point of a solution made using toluene in benzene is determined to be -130 C. Which has higher vapor pressure water or ethanol. Water bp 100 C benzene bp 80 C chloroform bp 61 C acetone bp 56 C explanation.
At 25C ether has the highest vapor pressure. A CsH2 b MgCl2 c CH-CH3NH2 d PBT e SOCI 4. A 200 g of glucose C6H12O6 in 1000 mL of water.
C 10 M solution of molecular compound sucrose C12 H22 O11. Which of the following aqueous solutions would have the highest vapor pressure. A 010 M NaCl B 010 M MgCl2 C 010 M CH3OH D 010 M Li3PO4.
A CH32N b CH OCH c CH-CH2OH d CHCHF 3. V P noof particlesions 1 Since glucose is a. Which of the following aqueous solutions would have the highest vapor pressure.
When comparing vapor pressures we need to be making comparisons at the same temperature. Hence from this we conclude that the pure water have the highest vapor pressure. What is the vapor pressure of a solution prepared by dissolving 250 g of C2H5OH in 375 g of.
Which of the following will have the highest boiling point at 1 atm pressure. Which of the following should have the lowest boiling point. Which of the following will have the highest boiling point at 1atm pressure.
The compound with the weakest intermolecular forces will have the highest vapor pressure. Which of the following should have the highest surface tension. 2 Get Similar questions.
Ether has the highest vapour pressure ie lowest boilint point at any temperature. Vapor pressure or vapour pressure in English-speaking countries other than the US. Which of the following will have the highest FP.
Both liquids have the strong dipole-dipole interaction called hydrogen bondingAcetone is polar but does not have H-bonding. It is much larger than water and thus experiences larger London forces. Which of the following has the highest vapor pressure.
Which of the following has the highest vapor pressure. Which one of the following solutions has the highest vapor pressure. The vapor pressure of water at 35 oC is 42175 mm Hg.
Which of the following has the highest vapor pressure. There are three puddles of different sizes on a sidewalk. Its vapor pressure is of intermediate value.
Which of the following would have the highest vapor pressure. When liquids evaporate the molecules must. See spelling differences or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases solid or liquid at a given temperature in a closed systemThe equilibrium vapor pressure is an indication of a liquids evaporation rate.
Water H2O100C methanol CH3OH6496C ethanol CH3CH2OH785C diethyl ether CH3OH2-O-CH2CH3 345C ethylene glycol HO-CH2-CH2-OH198C. B 200 g of sucrose C12H22O11 in 1000 mL of water. A 010 M NaCl B 010 M MgCl2 C 010 M CH3OH D 010 M Li3PO4.
A small puddle of rainwater a medium-sized puddle of spilled hot water a large puddle of spilled cold water which puddle has the highest vapor pressure. Which of the following would have the highest vapor pressure. The lowest boiling point will have the highest vapor pressure.
Which of the following will have the highest vapor pressure. Which of the following would have the highest vapor pressure. Which has the highest vapor pressure quizlet.
Thus at room temperature the substance with the lowest boiling point will have the highest vapor pressure easiest to. A CsH2 b CH24 c CHIA d C10H22 e C12H26 2. Of particles in solutionions.
B 10 M solution of ionic compound potassium chloride KCl. Vapour Pressure of equimolar solutions is inversely proportional to no. Pentane is a nonpolar substance and its vapor pressure is high compared to those of water and ethyl alcohol.
The freezing point of pure benzene is 549 C. Vapor pressure is a liquid property related to evaporation. As we know that the vapor pressure is depend on the Vant Hoff factor but the vapor pressure of a pure solvent is always greater.
Ethyl alcohol and water have very low vapor pressures. It also has the highest vapor pressure. At one atm pressure.
Which of the following should have the lowest vapor pressure. The substance with the highest boiling point will have the lowest vapor pressure. The reason for its high vapour pressure is that the attraction is less between ether molecules than between water and alcohol molecules.
CH3CH2CH2F is the largest and only polar compound and therefore has the greatest intermolecular forces both dipole-dipole and the greatest London Dispersion forces and is eliminated.
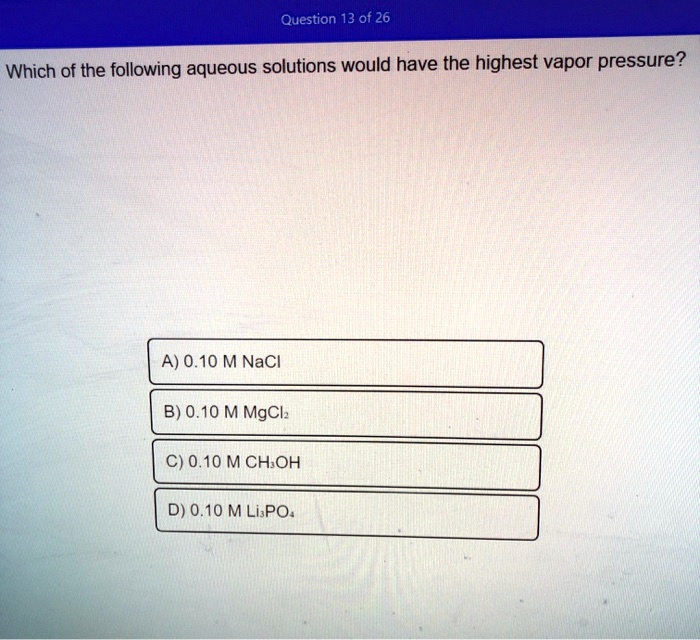
Solved Question 13 Of 26 Which Of The Following Aqueous Solutions Would Have The Highest Vapor Pressure A 0 10 M Nacl B 0 10 M Mgclz C 0 10 Mchoh D 0 10 M Lispo
Solved Which Of The Following Compounds Has The Lowest Vapor Chegg Com
Comments
Post a Comment